On November 13, 2023, Verve Therapeutics shared positive early Phase I results from the first in vivo base editing human trial, demonstrating “Dose-Dependent Reductions in LDL-C and Blood PCSK9 Protein in Patients with Heterozygous Familial Hypercholesterolemia”.
So, what is ‘base editing’?
Base editing, along with CRISPR-Cas9, are two common methods of gene editing that permanently change the genetic code in DNA sequences within cells. They are somewhat different. In the case of CRISPR-Cas9, gRNA is used to identify the target sequence, and the Cas9 protein enzyme functions like a pair of scissors, cutting both strands of DNA, leading to double-strand breaks (DSBs). DSBs can sometimes result in off-target effects. In base editing, the CRISPR-Cas9 system is also utilized to scan DNA and identify the target sequence. However, it specifically aims to change a single nucleotide within the double-helical DNA structure using a specialized base editing enzyme called deaminase, which carries out the chemical modification and is fused to the Cas9 protein. Base editing can be employed to either silence or activate a specific gene.
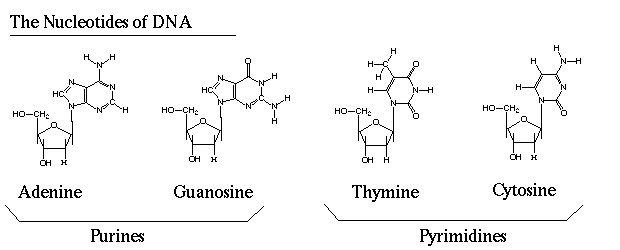
Although base editing can eliminate the creation of DSBs and the associated off-target effects, it has its own limitations. One of the major drawbacks is its limited capability to modify DNA. So far, only two major classes of base editors have been developed: cytidine base editors (CBEs), which convert C to T, and adenine base editors (ABEs), which convert A to G. The four nucleotides of DNA are depicted in Figure 1.
Verve’s delivery system utilizes GalNAc-LNP, where the GalNAc ligand is added to the surface of the LNP. Several siRNA companies also conjugate their siRNA molecules with GalNAc for targeted delivery, as discussed in a previous blog post siRNA companies. The GalNAc ligand targets the asialoglycoprotein receptor (ASGPR), which is highly expressed in mammalian hepatocytes (liver cells). However, other organs may also express ASGPR protein weakly, such as the gallbladder and stomach.
Returning to the Phase I study, it’s a small-scale study in which only nine patients were treated with different doses of VERVE-101. Patients treated at potentially therapeutic doses (0.45 mg/kg and 0.6 mg/kg) experienced a significant decrease in blood PCSK9 protein and low-density lipoprotein-cholesterol (LDL-C). The results are promising, but the occurrence of two serious adverse events raises concerns about the safety of this new therapeutic approach. One patient had a fatal cardiac arrest approximately five weeks after treatment (which was later determined to be unrelated to the treatment), and another experienced a myocardial infarction the day after treatment. These incidents highlight the need for more data to address the safety aspects of this innovative therapy.
Reference: