Analytical procedures are applied to ensure the identity, strength, quality, purity, and potency of each drug substance and drug product manufactured for human use.
Analytical test methods can be used to guide process development, evaluate API stability, guide formulation design, release starting materials (SMs)/intermediates, API release, and in process control.
API characterization tests include:
- Appearance
- Identification
- Content purity
- Organic impurities
- Inorganic impurities
- Volatile content
- Counterion content
- Potential genotoxic impurities
- Particle size
- Solid-state characterization
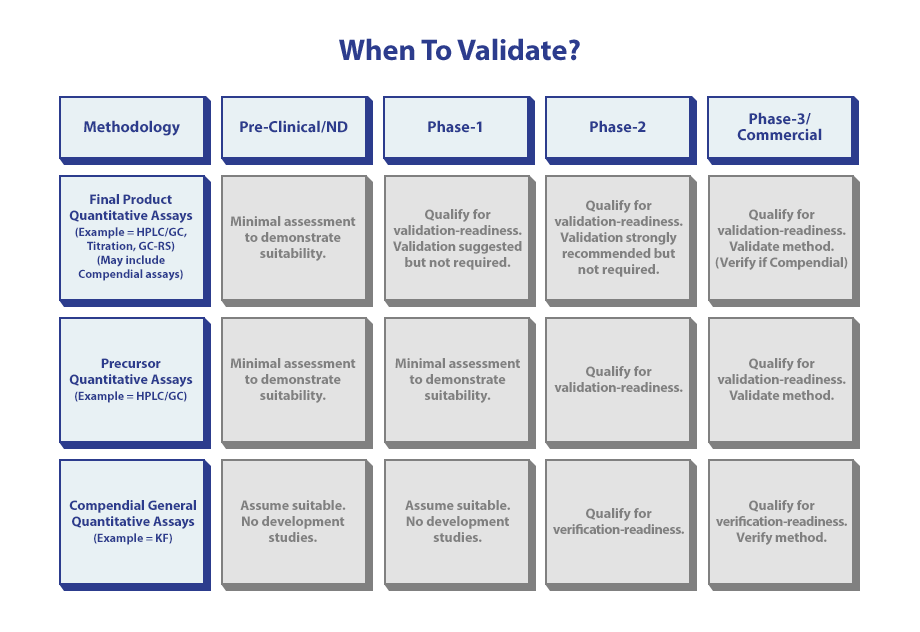
Stage Goals:
- Preclinical: Assign a purity level to the drug substance (DS) used for toxicology dosing studies and qualify impurities present in the API in animal studies.
- FIH/Phase 1: Assign purity to DS for First-in-Human (FIH) or safety studies and evaluate impurity levels.
- Phase 2: Assign purity to DS for a larger patient population (efficacy clinical studies) and evaluate impurity levels.
- Phase 3: Develop a well-characterized API for pivotal clinical studies and control impurity levels to meet proposed commercial targets.
- Commercial: Ensure that all API critical quality attributes meet regulatory standards (e.g., ICH).
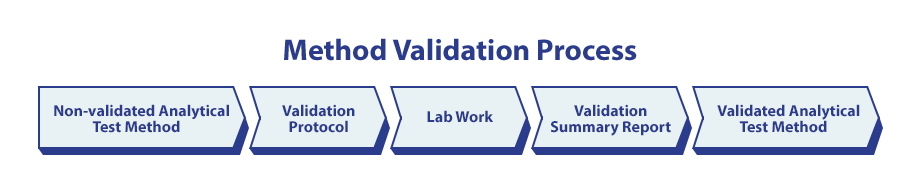
Method validation: Analytical method validation, a protocol-guided activity, ensures that a test procedure is accurate, reproducible, and sensitive within a specified range based on the application of the method. It assesses an analytical method to demonstrate its suitability for the intended purpose by evaluating performance characteristics against pre-approved acceptance criteria.
Method qualification: This shows that a method is suitable for use based on the evaluation of specific performance characteristics. Method qualification is often used to describe methods that have been scientifically proven to be appropriate for early-phase drug development (e.g., pre-clinical, Phase I).
Method verification: A guided demonstration that proves a compendial method is suitable for use in a particular environment or quality system (e.g., equipment, personnel, facility, etc.).
Method transfer: A process in which an analytical method is formally moved from a sending laboratory to a receiving laboratory. Method transfer is similar to method validation but also includes comparative assessments and criteria demonstrating performance between laboratories (see USP <1224>).
Performance characteristics:
- Specificity: The ability to assess unequivocally the analyte in the presence of other components that may be expected to be present.
- Sensitivity (LOQ/LOD): Limit of Detection (LOD) is the lowest amount of analyte in a sample that can be detected but not necessarily quantitated precisely. Limit of Quantitation (LOQ) is the lowest amount of analyte in a sample that can be quantitatively determined with suitable precision and accuracy.
- Accuracy: Also referred to as trueness, it expresses the closeness of agreement between the value accepted as a reference value and the determined value.
- Precision: The closeness of agreement between a series of measurements obtained by multiple samplings, including repeatability, intermediate precision, and reproducibility.
- Linearity: The ability to obtain test results in a set interval that are directly proportional to the amount of the analyte in a test solution. It is related to the range.
- Range: The interval between the upper and lower amounts of an analyte in an analytical procedure that has demonstrated an acceptable degree of precision, accuracy, and linearity.
- Robustness: A measure of the capacity of an analytical procedure to remain unaffected by small, deliberate variations in method parameters, providing an indication of its reliability during normal usage.
Source: