Sugars and lipids are two distinct classes of biomolecules found in living organisms, each with its own unique chemical composition, structure, and functions. Today, we will focus solely on the differences in their chemical structures.
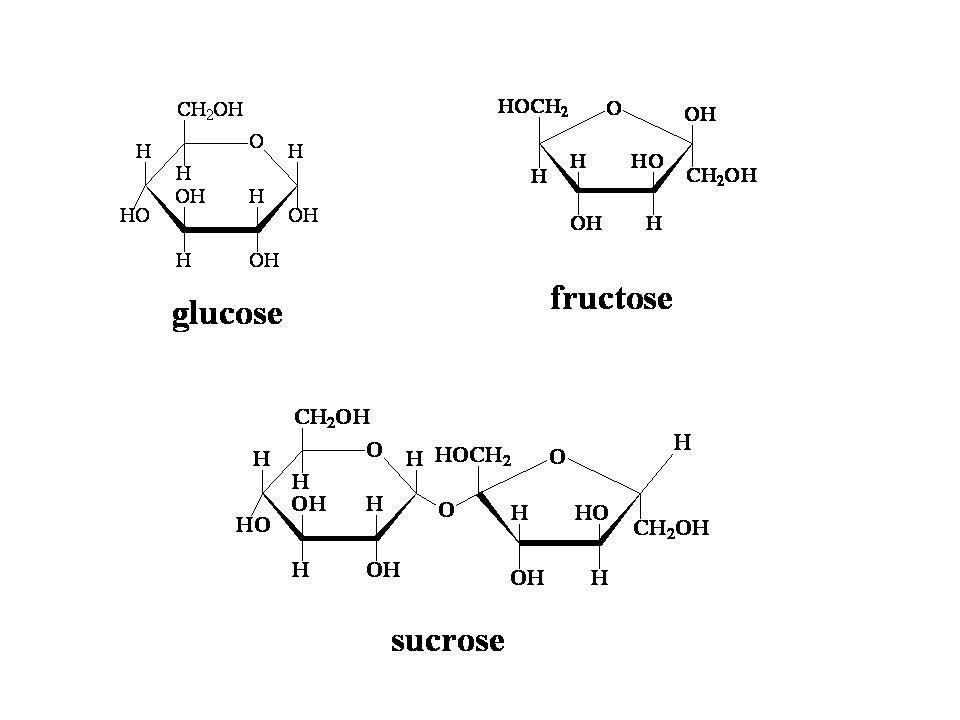
Sugars are organic compounds composed of carbon (C), hydrogen (H), and oxygen (O) atoms. Their basic building blocks are monosaccharides (single sugar units) like glucose and fructose (as shown in Figure 1).
Lipids are also organic compounds primarily composed of carbon (C), hydrogen (H), and oxygen (O) atoms. They form a broad class of organic molecules that include various compounds, such as triglycerides (fats and oils), phospholipids, steroids, and waxes. Lipids often contain fatty acids as one of their components. Let’s examine the structure of each of these individual compounds.
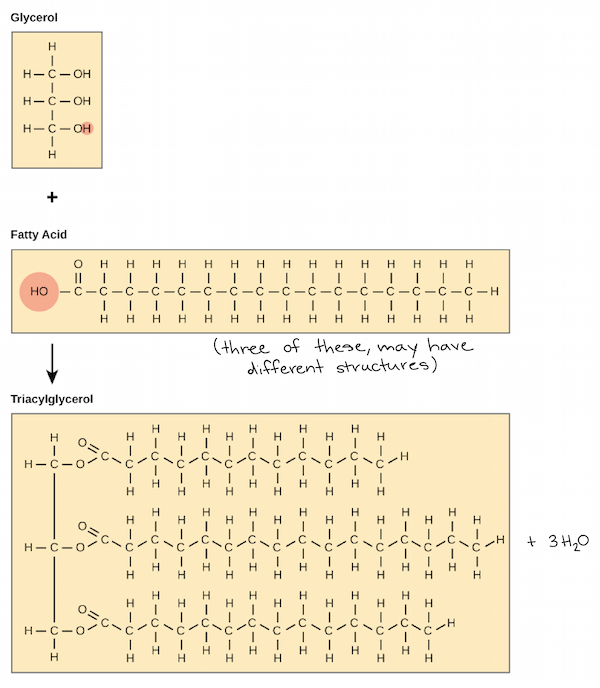
Fats encompass both solid fats (saturated fats) and liquid fats (oils, unsaturated fats). In this discussion, we will focus on solid fats only. Fats are typically composed of triglycerides, which consist of three fatty acid molecules attached to a glycerol backbone (as shown in Figure 2). The composition of fats can also include other lipids, such as phospholipids and cholesterol, depending on the specific type of fat.
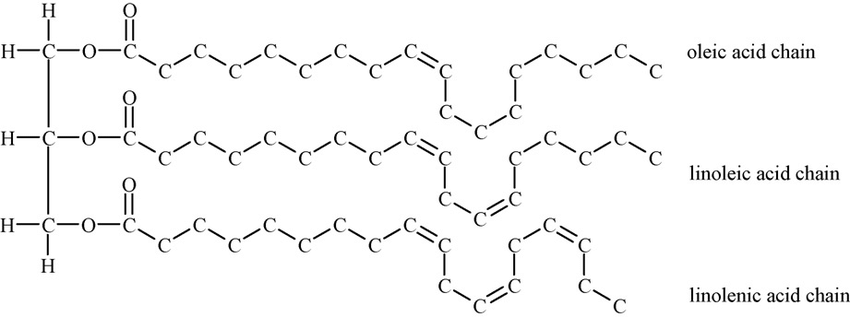
Oils (also known as liquid fats or unsaturated fats) are a type of fat, and their liquid state primarily results from the presence of unsaturated fatty acids. Typically, they are composed of triglycerides, similar to solid fats, but they have a higher proportion of unsaturated fatty acids (as depicted in Figure 3). Unsaturated fatty acids are fatty acid molecules that have one or more double bonds between the carbons in their hydrocarbon chain. As a result, the chain contains fewer hydrogens than the maximum possible.
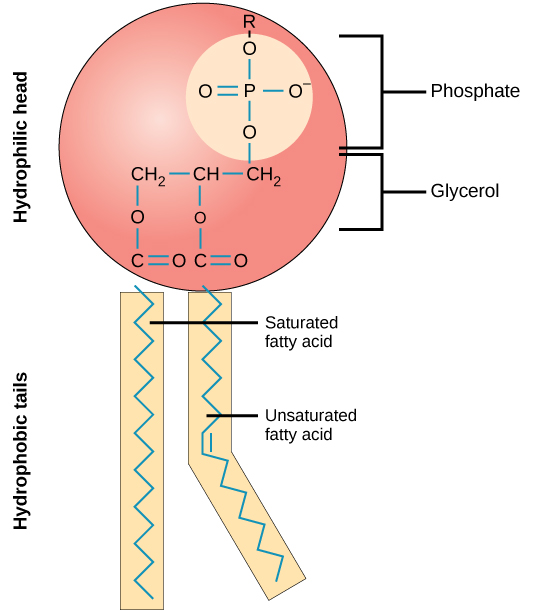
Phospholipids are composed of several components, including a phosphate head group, a glycerol backbone, and fatty acid tails. The R group in the phosphate head group is what renders the head hydrophilic (as shown in Figure 4).
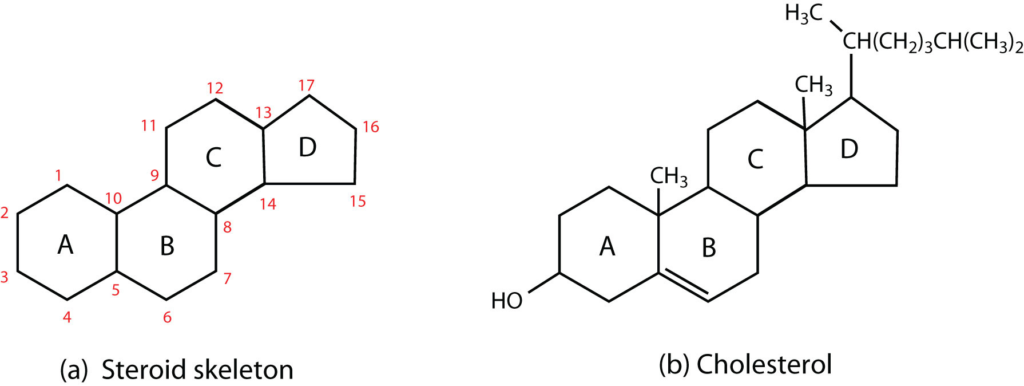
Steroids are all characterized by a distinctive molecular structure consisting of 17 carbon atoms, organized into four rings conventionally denoted by the letters A, B, C, and D. These carbon rings are bonded to 28 hydrogen atoms, as illustrated by the steroid skeleton in Figure 5(a). In addition to the four-ring structure, steroids may have side chains or functional groups attached to the core structure. One of the most well-known steroids is cholesterol (depicted in Figure 5(b)).
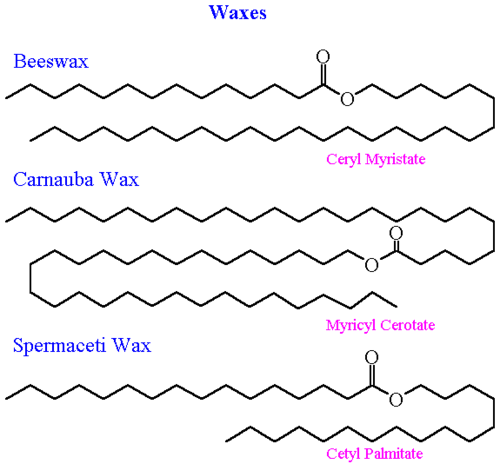
Waxes have a fundamental structure consisting of long-chain fatty acids esterified with long-chain alcohols, as represented in Figure 6.
Reference:
- https://cdavies.wordpress.com/2009/01/27/simple-sugars-fructose-glucose-and-sucrose/
- https://www.khanacademy.org/science/biology/macromolecules/lipids/a/lipids
- https://www.researchgate.net/figure/Molecular-structure-of-a-vegetable-oil-example-applies-for-rapeseed-oil-All-double_fig1_223370968
- https://pressbooks.calstate.edu/nutritionandfitness/chapter/6-2-phospholipids-and-sterols/
- https://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/s20-04-steroids.html
- https://quizlet.com/24300613/chem-unit-15p-intro-to-organic-biochemistry-flash-cards/