The basic building blocks for DNA or RNA are nucleotides. However, there are also other terms that are often confusing. We’ll take a look at the building blocks of DNA/RNA and clarify the difference between nitrogenous base, nucleoside, and nucleotide.
Figure 1 shows the nucleotide structure. It consists of three parts: the nitrogenous base (highlighted in yellow), the five-carbon sugar (highlighted in blue), and a phosphate group(s) (highlighted in orange). Nucleoside, on the other hand, only contains a nitrogenous base and a five-carbon sugar, lacking phosphate group(s). Therefore:
Nitrogenous base
Nucleoside = nitrogenous base + sugar
Nucleotide = nitrogenous base + sugar + phosphate group(s)
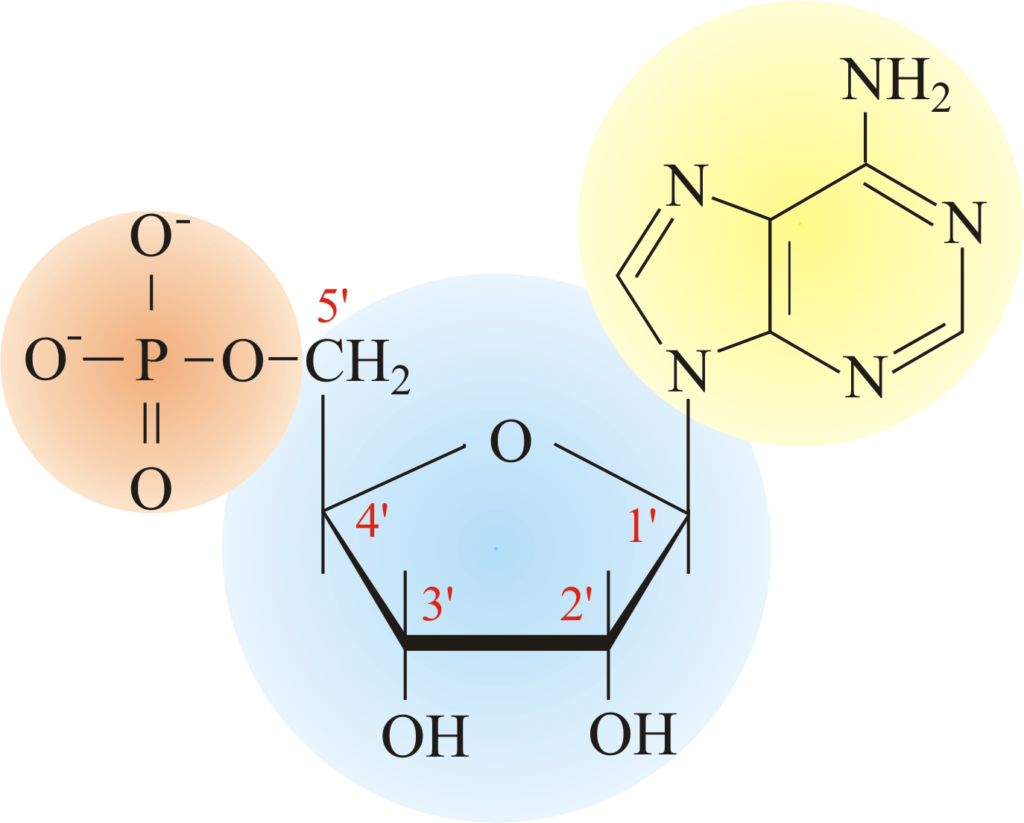
- Nitrogenous base
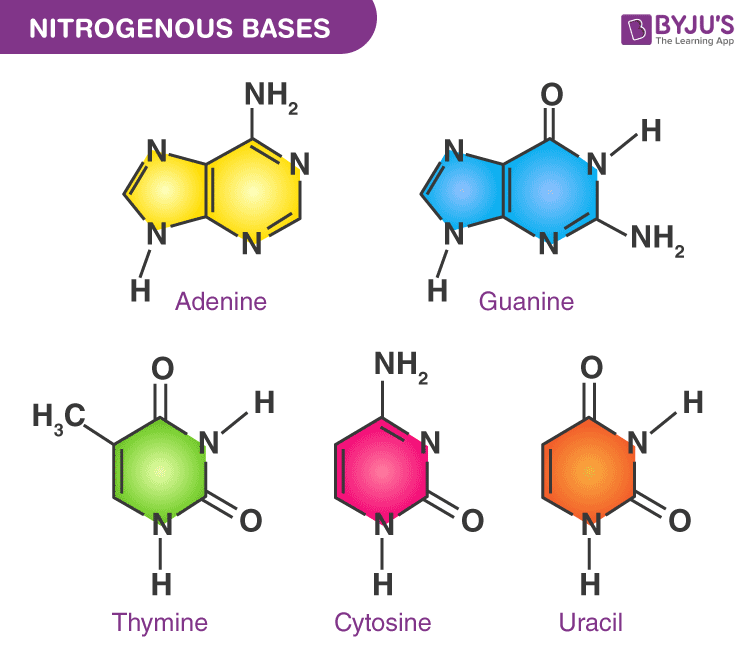
There are a total of five naturally occurring nitrogenous bases: Adenine (A), Guanine (G), Thymine (T), Cytosine (C), and Uracil (U) (See Figure 2). Depending on how many rings are present in the base, there are two categories: purines (2 rings) and pyrimidines (1 ring). Therefore, A and G are purines, and the rest three are pyrimidines. In a DNA molecule, only four bases are present: A, G, T, and C, and they can form two base pairs of Watson-Crick hydrogen bonding: A-T and C-G. The pairing of bases is what makes the double-helix structure of DNA. In an RNA molecule, T is replaced by U. The base pairing in RNA molecules is A-U and C-G.
- Sugar molecule
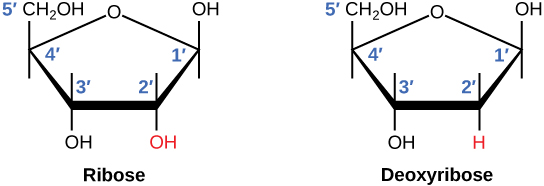
In a nucleotide, there is a four-carbon sugar molecule between the nitrogenous base and phosphate group(s) (shown in Figure 3). The ring is formed by four carbon atoms and one oxygen atom, and the fifth carbon atom is outside of the ring. The carbon atoms are numbered from 1′-5′ clockwise in order. Therefore, 1′-carbon is connected with the nitrogenous base, and 5′-carbon is connected to phosphate group(s). The only difference between the carbon molecule of DNA and RNA lies in the 2′-carbon. In DNA, 2′ is a deoxy hydroxyl group, with only a hydrogen atom left, while for RNA, 2′ is a hydroxyl group. Therefore, the names of sugar molecules are Ribose and Deoxyribose in RNA and DNA respectively.
- Phosphate group(s)
The last part of a nucleotide is phosphate group(s). One or more phosphate groups are esterified to the 5′ carbon of the sugar molecule. The most commonly used phosphates are monophosphate (one phosphate group), diphosphate (two phosphate groups), and triphosphate (three phosphate groups). Then the acronyms of the nucleotides are:
Monophosphate nucleotides – AMP, TMP, CMP, GMP, UMP
Diphosphate nucleotides – ADP, TDP, CDP, GDP, UDP
Triphosphate nucleotides – ATP, TTP, CTP, GTP, UTP
For a complete summary of names for nitrogenous bases, nucleosides, and nucleotides, please see Table 1.
| Base | Ribonucleoside | Ribonucleotide | Deoxyribonucleoside | Deoxyribonucleotide |
| Adenine (A) | Adenosine | AMP, ADP, ATP | Deoxyadenosine | dAMP, dADP, dATP |
| Guanine (G) | Guanosine | GMP, GDP, GTP | Deoxyguanosine | dGMP, dGDP, dGTP |
| Thymine (T) | N/A | N/A | Deoxythymidine | dTMP, dTDP, dTTP |
| Cytosine (C) | Cytidine | CMP, CDP, CTP | Deoxycytidine | dCMP, dCDP, dCTP |
| Uracil (U) | Uridine | UMP, UDP, UTP | N/A | N/A |
Reference: